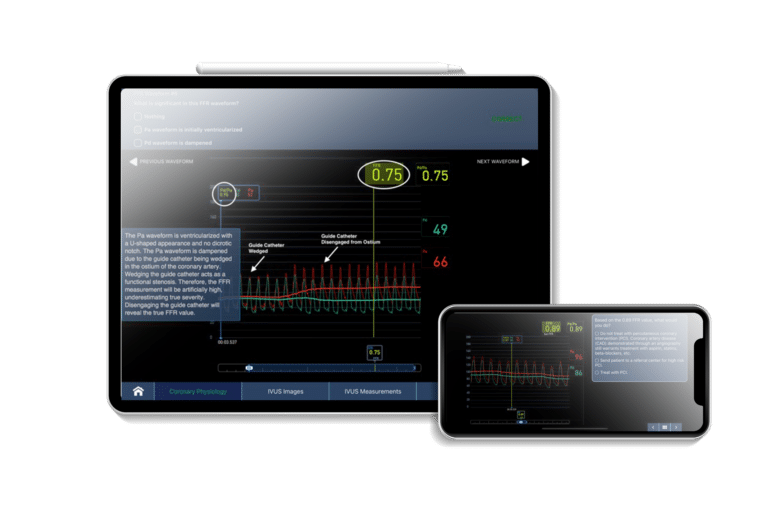
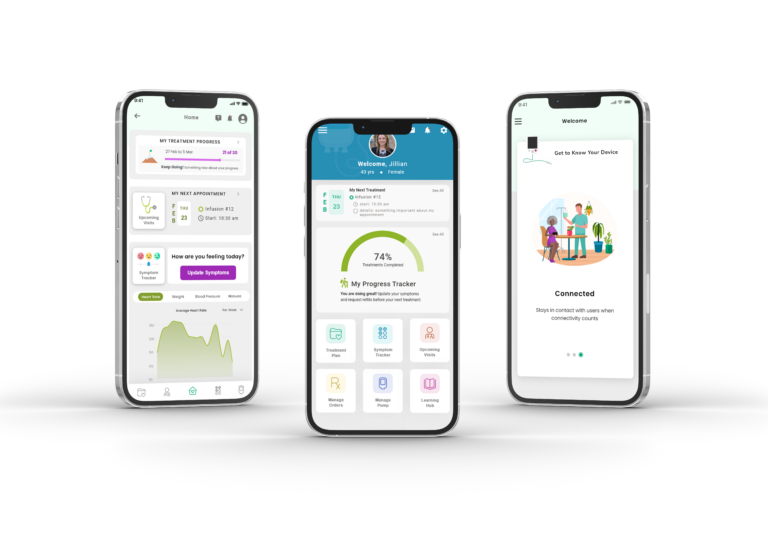
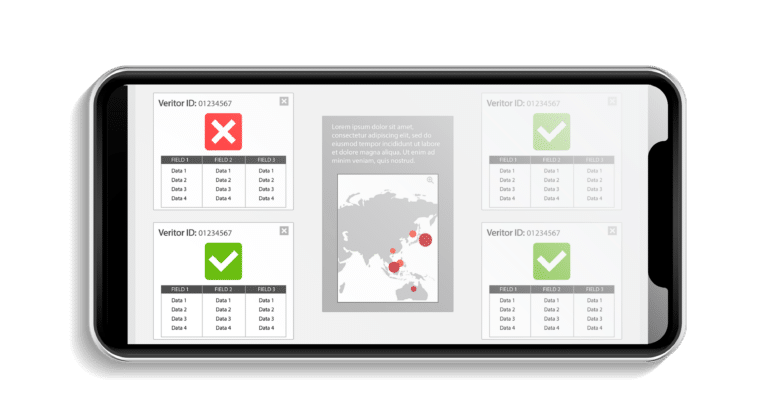
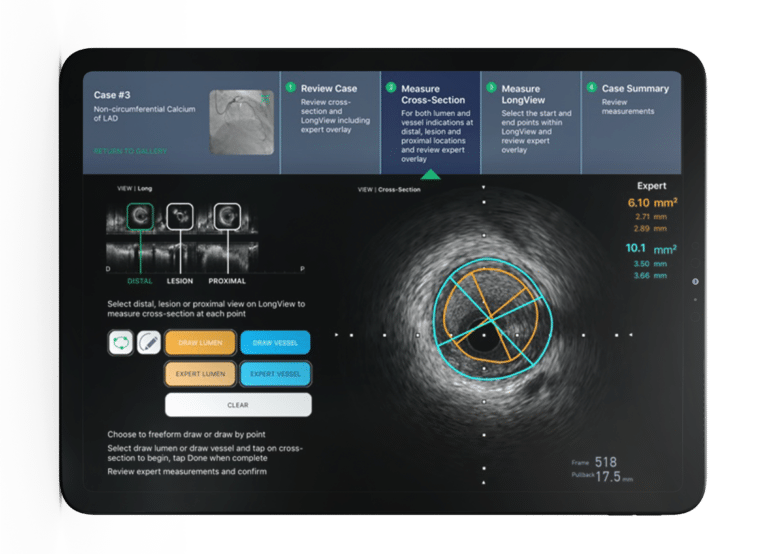
Digital Health Software Development Projects
As technology continues to advance all facets of healthcare, software has become a key differentiating component of medical products.
At Syncro Medical we design, build, and scale the enabling software for our client’s market-leading devices. Our Digital Health team brings extensive experience in the core technologies underlying Digital Health, resulting in an accelerated time to market and optimization of resources.
Syncro Medical provides critical expertise to companies seeking to transform their products with digital technologies.
Our extensive expertise in software development, our focus on quality, and our optimized project management methodologies, enable us to address the specific needs of your project and create world-class software for any medical device.




DIGITAL HEALTH PROJECTS