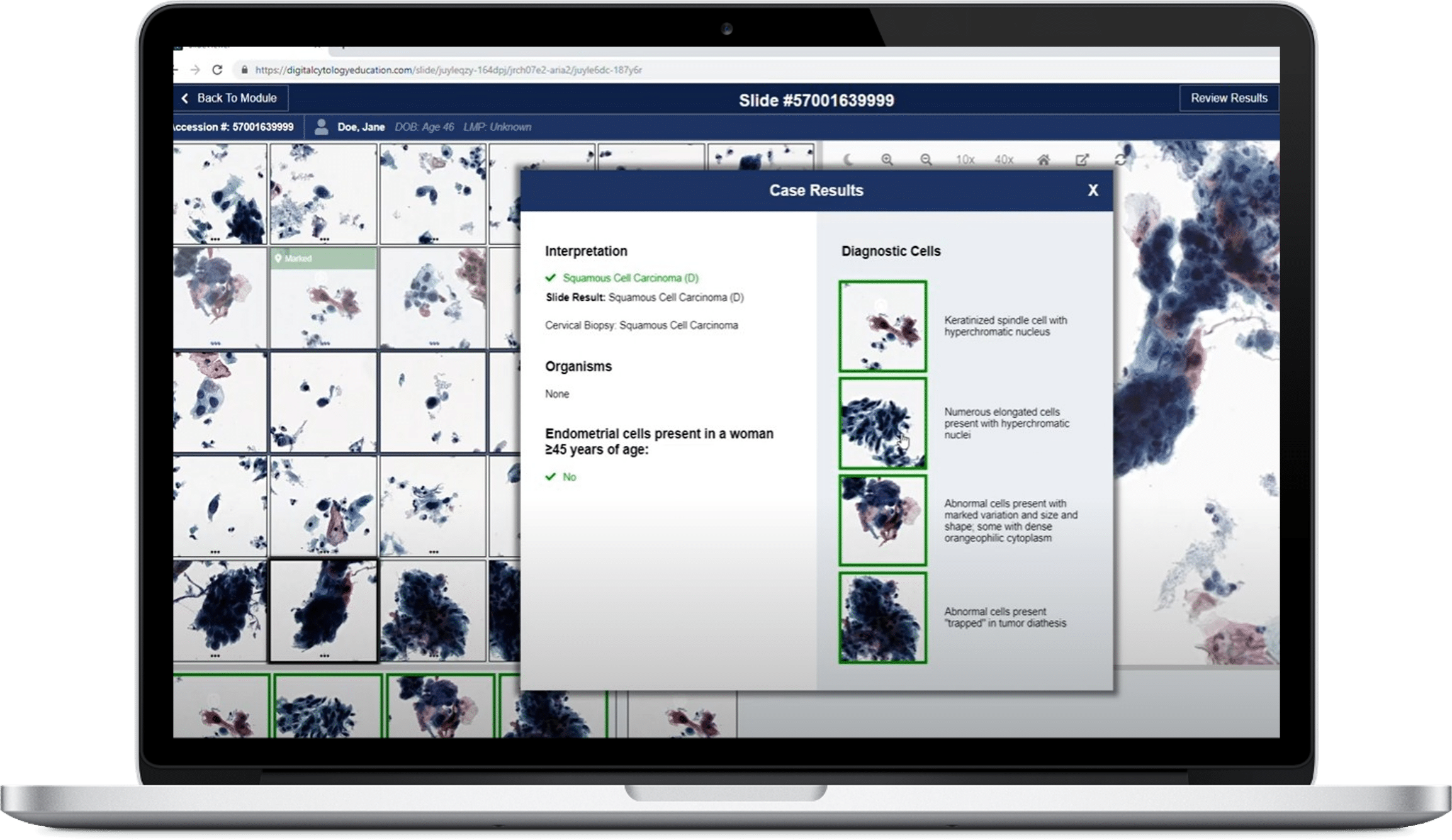
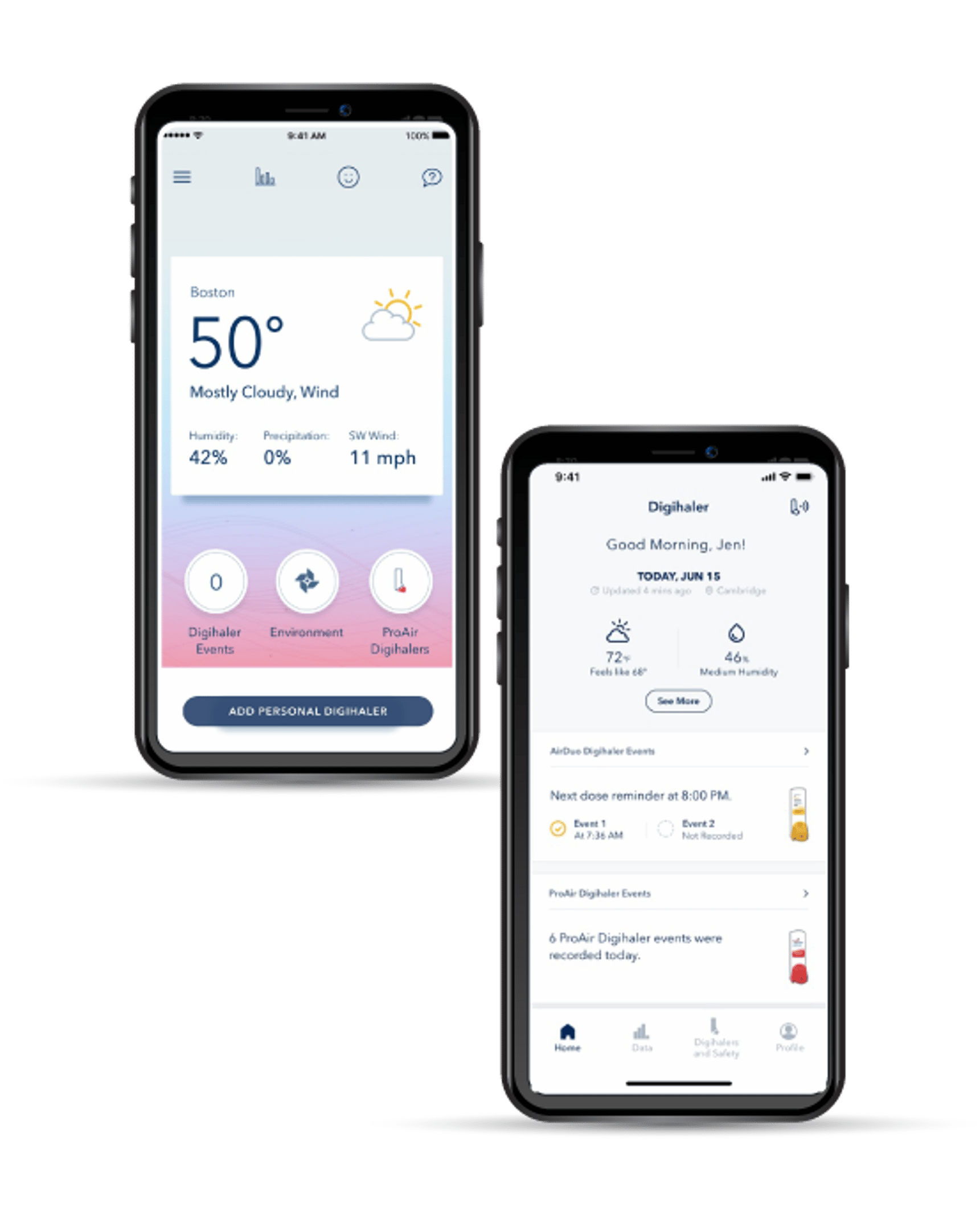
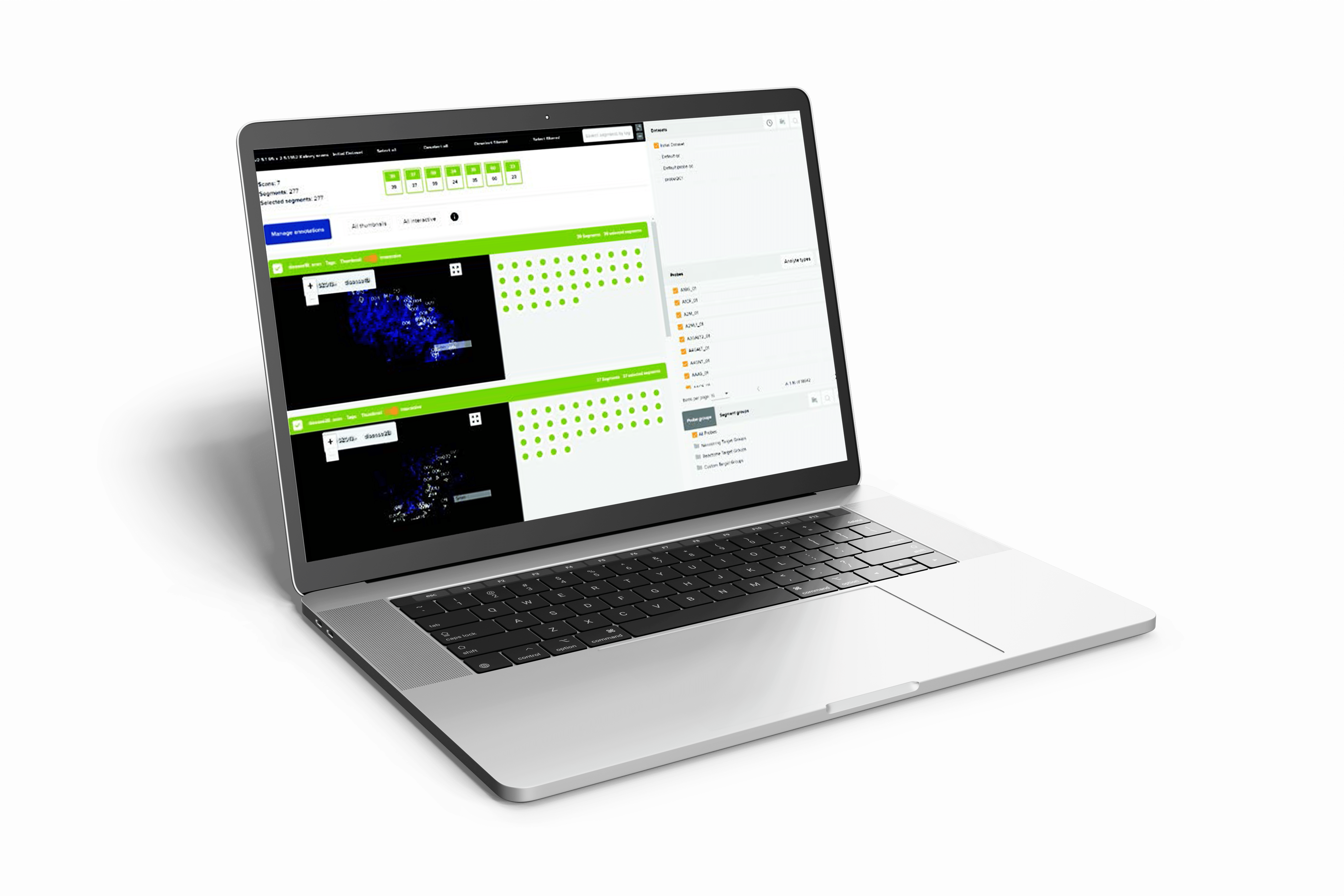
Developing medical device software requires addressing numerous challenges related to regulation, integration, usability, security, testing, and quality assurance.
Without a solid understanding and commitment to the defined quality systems and development processes necessary for medical device software, the resulting code can’t stand up to regulatory scrutiny.
For more than 30 years, Syncro Medical has focused solely on developing the software components for our client’s products.
Our best-in-class teams include software architects, UX/UI designers, developers, and quality assurance experts. These highly specialized teams understand the lifecycle of software products resulting in more efficient development and faster time-to-market for our clients.