Decades of Collaboration. Hundreds of Devices. One Trusted Partner.
Where Medtech Turns for Trusted Software Development
HOW WE CAN HELP
Medical Device Design and Development
We partner with medical device R&D teams to transform complex ideas into fully realized, regulatory-ready software.
Whether you need end-to-end development or specialized expertise in UI/UX, cloud, mobile, cybersecurity, or embedded systems, Syncro Medical brings the experience and engineering excellence to move your product forward.
Having completed hundreds of projects for clients ranging from start-ups to Fortune 500 companies, we’ve worked in virtually every segment of Medtech.
Leveraging Agile development methodologies, human-centered design principles, and adhering to industry standards around security and risk management, we help our clients at every stage of the software development lifecycle.
We transform complex technologies into reliable software—accelerating innovation through secure data integration and connectivity across devices, apps, & the cloud.
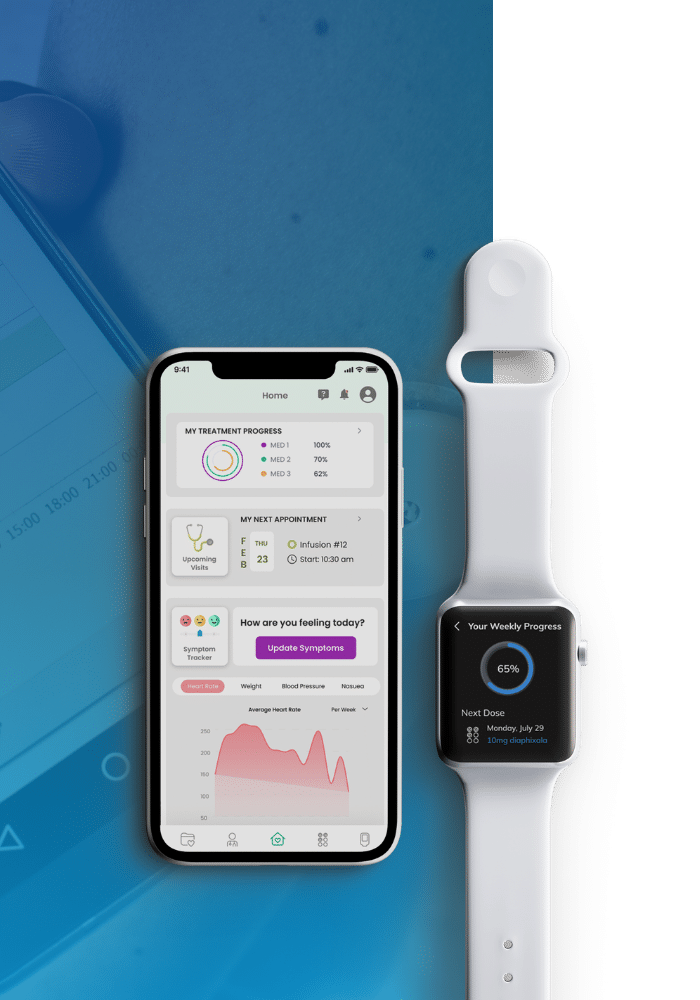
We Transform Complex Technology into Innovative Medtech Products
Emerging technologies like AI, IoT, and advanced digital technologies are transforming what medical devices can do — from predicting patient risk to enabling continuous monitoring and cloud-based insights. But successfully integrating these technologies into regulated products requires deep technical expertise and a disciplined approach.
That’s where Syncro Medical comes in –
For over 30 years, we’ve helped leading MedTech companies turn complex technologies into reliable, compliant, and high-performing software. Whether enhancing an existing system or building next-generation devices, our engineers ensure your technology works seamlessly, scales effectively, and meets the highest standards of safety and performance.
WHAT OUR CLIENTS SAY
A Proven Medtech Software R&D Partner
Syncro Medical has consistently maintained an outstanding reputation in the industry. Across hundreds of projects, nearly 100% of our clients strongly agree that Syncro Medical provides significant value to their business.
Our flexible, collaborative approach to software project management ensures that our clients achieve not only fast time-to-market but superior product functionality, quality, and scalability as well.
Always flexible, always scalable, and they always deliver on time and on budget. Would recommend to anyone, and hope to continue our partnership moving forward.”

Each team member was extremely knowledgeable about the portion of the development process that they owned, if changes needed to happen, they were handled very smoothly, and the entire process was a collaborative partnership.”

They have brought much-needed software development assistance to multiple efforts of ours. In a relatively short amount of time, we have come to rely on them as valued development partners - a critical part of our team - supplementing some of our most complicated and “visible” work. I look forward to continuing the relationship with Syncro Medical and forging an even stronger bond by working together in an even greater capacity. “

We now utilize Syncro Medical strategically to keep our own internal staff focused on our core technologies. Syncro Medical has helped us manage project risks, by off-loading tasks during our peak-demand development phases.”

Having them dedicate one of their team members as a project manager – a single point of contact for our software leads– has proven invaluable."