CERTIFIED: ISO 9001 | ISO 13485 | ISO 27001
CERTIFIED: ISO 9001 | ISO 13485 | ISO 27001
EMBEDDED DEVELOPMENT | WEARABLE DEVICE
Wearable Defibrillator
PROJECT OVERVIEW
Our client makes a wearable medical product for patients who are at risk for Sudden Cardiac Death. The device has an embedded controller with a touch interface.
They are working on a next-generation product and were looking for a software partner who could assist with updating the software for the wearable device and the corresponding monitor.
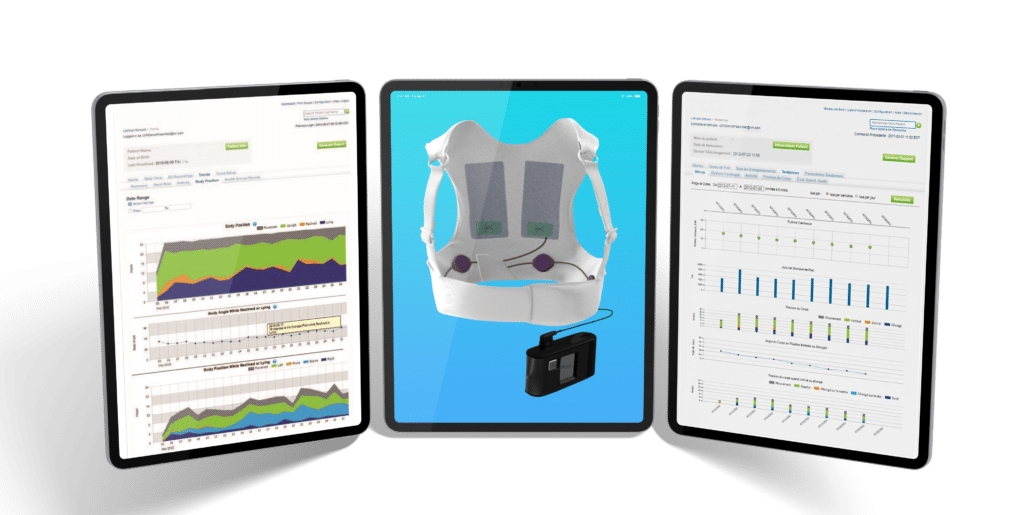
Syncro Medical led the next generation development efforts including:
- Updating software architecture & refactoring legacy code to fix issues and mitigate inefficiencies
- Updating embedded Linux-based monitor software to use Amazon Web Services (AWS) for data management
- Developed a prototype to extend the capabilities of the existing product to facilitate patient-directed rehab through a walking program
