Medical Device Proof-of-Concept
When you’re developing a new or next-generation medical device, the biggest hurdle is often demonstrating technical feasibility and securing stakeholder buy-in—before committing to costly full-scale development. Without a compelling proof-of-concept, it’s easy to waste time and resources on unproven ideas that fail to meet clinical needs, investor expectations, or regulatory requirements.
We help medtech innovators quickly build robust software proofs-of-concept that validate key features, de-risk critical technologies, and generate the evidence needed to secure funding, align internal teams, and plan your path to commercialization.

Learn how our proof-of-concept services can help you move forward with confidence.
SERVICES
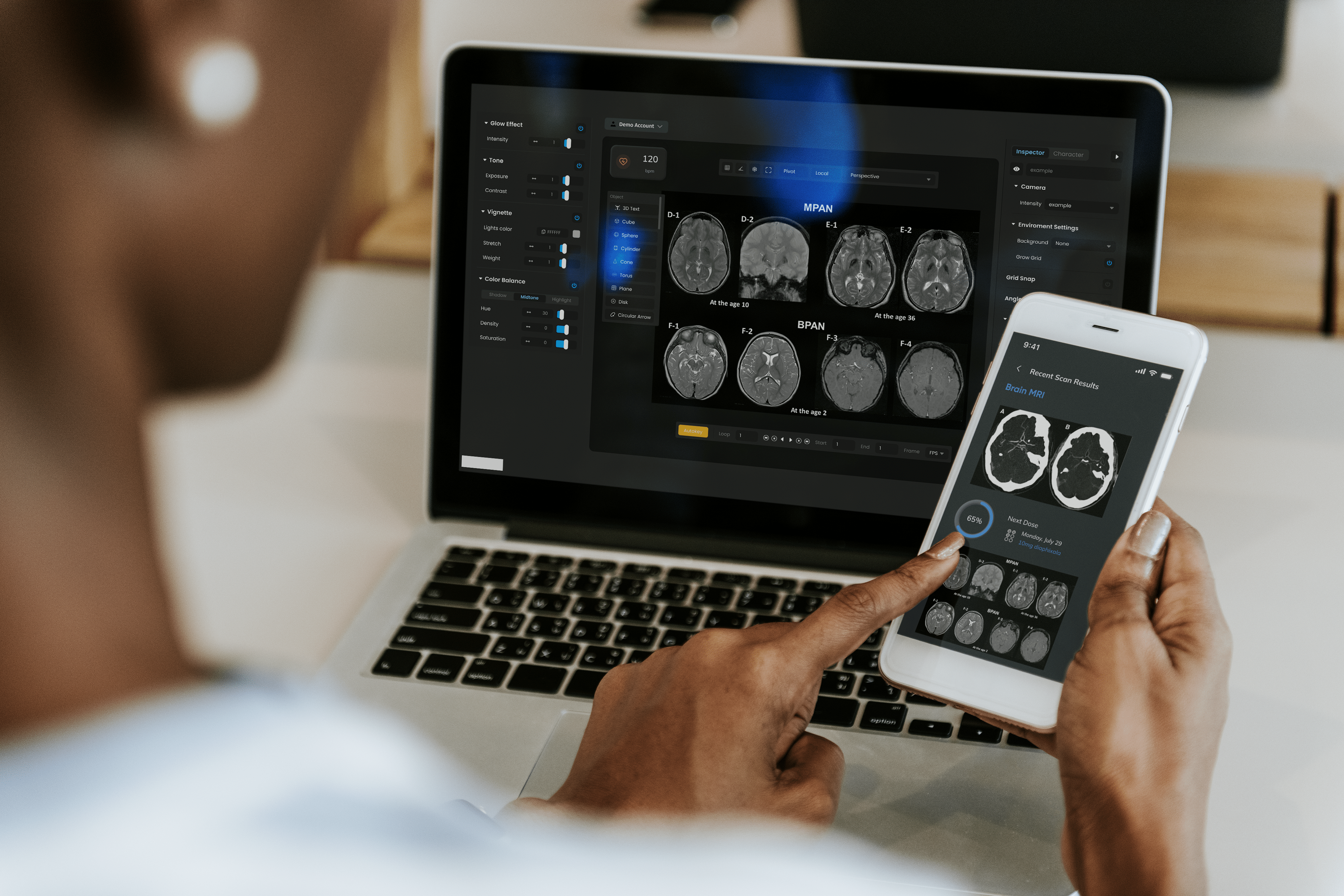
Medical Device Proof of Concept Development Services
The first step in moving your product from mind to market is developing a medical device proof of concept.
A proof of concept will enable your team to evaluate and select the best application areas and markets for your technology.
At Syncro Medical we are uniquely positioned to guide you through the proof of concept development process. Our team of business analysts, researchers, system architects and engineers help our clients assess, test, and select the best pathway to commercialization.
TECHNICAL FEASIBILITY
Our Business Analysts, UX Strategists, and Software Architects conduct quantitative and qualitative studies to assess, refine, and optimize your technology. Conducting a technical feasibility will reduce risks, increase efficiency, minimize development costs, and avoid having to rebuild in future phases.
USABILITY RESEARCH
Human-Centric UX-UI design is an integral approach to creating products that solve specific users’ needs. Our UX strategists can help to pinpoint the users’ pain points, desires, and expectations that result in products that meet their real-world needs.
RAPID PROTOTYPING
Prototyping is a fundamental stage in our development process.
Using Proof-of-Concepts or clickable and interactive prototypes our team quickly gets your device in the hands of your target users to test and validate your product vision.

Can a medical device proof of concept (POC) help validate your ideas and ensure that your product meets market needs before full-scale development? Absolutely.
By following these principles, you can ensure that your POC remains efficient, focused, and useful for addressing key questions before moving into full-scale development.
Ensure that your medical device proof of concept (POC) is successful by avoiding these common pitfalls:

1. SET CLEAR GOALS
Without clear goals, the POC can drift and lose focus. Defining the objectives early on allows for more structured and effective progress, though goals can evolve as necessary.
HOW WE HELPED
2. STICK TO SHORT TIMELINES
A POC should be fast and iterative, focusing on quick learning and adjustments. Keeping the POC phase short prevents it from merging into broader development.
HOW WE HELPED


3. AVOID INVOLVING TOO MANY STAKEHOLDERS
Involving too many people at different stages can lead to delays and confusion. Keep the team small and focused on the specific goals of the POC.
HOW WE HELPED
4. MANAGE EXPECTATIONS
It's important to communicate that a POC is not the final product. Sometimes, stakeholders may assume that because a POC works or looks complete, the product is ready.
HOW WE HELPED


5. AVOID MIXING POC WITH PRODUCT DEVELOPMENT
Trying to reuse or merge the POC directly into the product development phase can lead to confusion. The POC is for rapid discovery, while product development requires a more structured approach.
HOW WE HELPED
DEVELOPMENT EXPERTISE
We support our clients at every stage of the medical device software design and development process
Learn more about how we help our clients accelerate software R&D for their market-leading devices.
