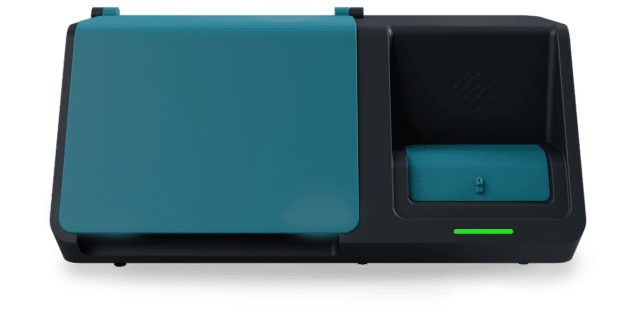
Life Science Software Development Projects
Leading manufacturers of diagnostic and life science instruments have come to rely on Syncro Medical to accelerate time-to-market for their new and enhanced products. With a singular focus on developing the software that powers these instruments, Syncro Medical’s elite software engineering teams bring rapid start-up, deep experience, and quality craftmanship to each client project.
We would welcome the opportunity to meet with you to discuss your next development project and how we can make your product concepts a reality.




LIFE SCIENCE & DIAGNOSTIC DEVICE PROJECTS

UX Design, Software Architecture & Development
Hematology Analyzer & Systems
PROJECT OVERVIEW
<b
A...
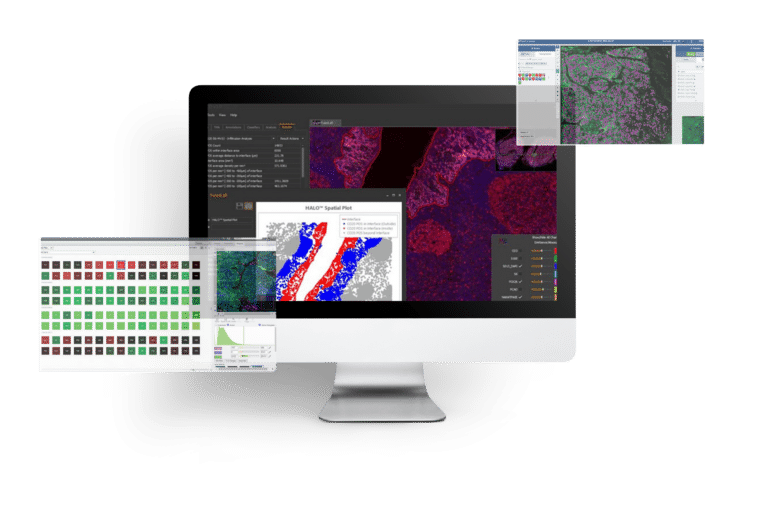
IMAGE PROCESSING & ANALYSIS
Multiplexed Imaging Solution for Spatial Biomarker Analysis
PROJECT OVERVIEW
Our...

DIAGNOSTIC INSTRUMENT
Multiplexed Digital Spatial Profiler
PROJECT OVERVIEW
Our client develops products...

Analyzer | Microfluidics
Point of Care Diagnostic Device
PROJECT OVERVIEW
A manufacturer in the respiratory...
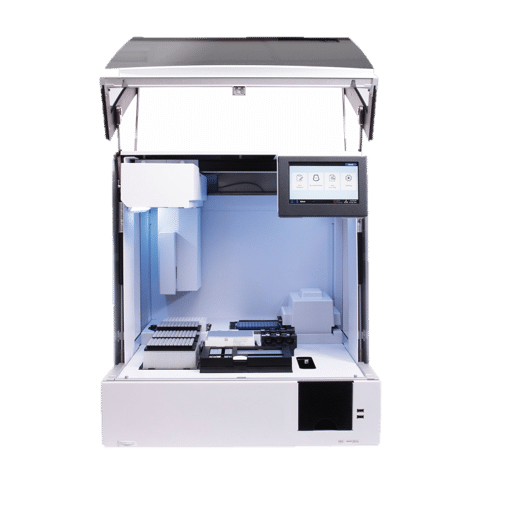
Early Stage Support for Diagnostic Instrument
Next-gen DNA Sequencing
| NGS Platform
PROJECT OVERVIEW
An...

AI/ML ENABLEMENT
AI-Powered Digital Diagnostic System
PROJECT OVERVIEW
FDA-cleared digital cytology system...

DIAGNOSTIC INSTRUMENT
Rapid AST Diagnostic Testing & Analyzer Platform
PROJECT OVERVIEW
Our client...
AI and Medical Imaging
AI-Enabled Ultrasound
PROJECT OVERVIEW
A portable AI-enabled ultrasound system...
No posts found