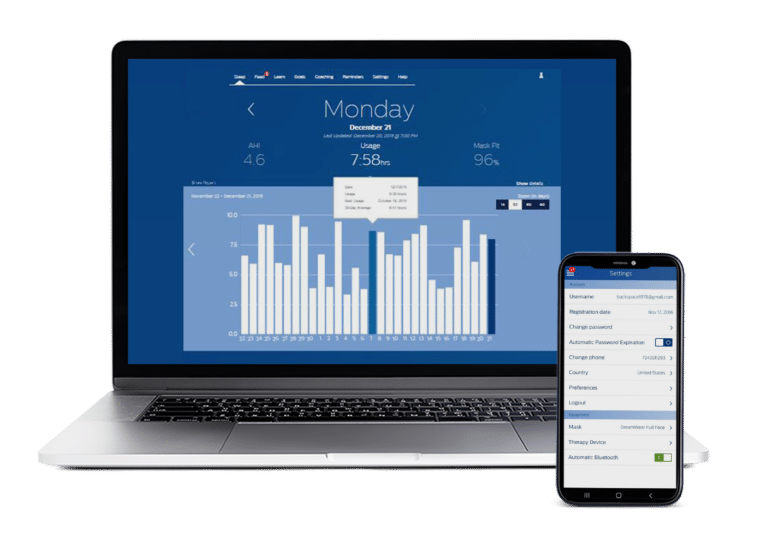
How We Helped
Syncro Medical designed and implemented the software for the device as well as the PC application and cloud-hosted system. The software is used by the patient and clinician to enable the therapy and to review device usage.

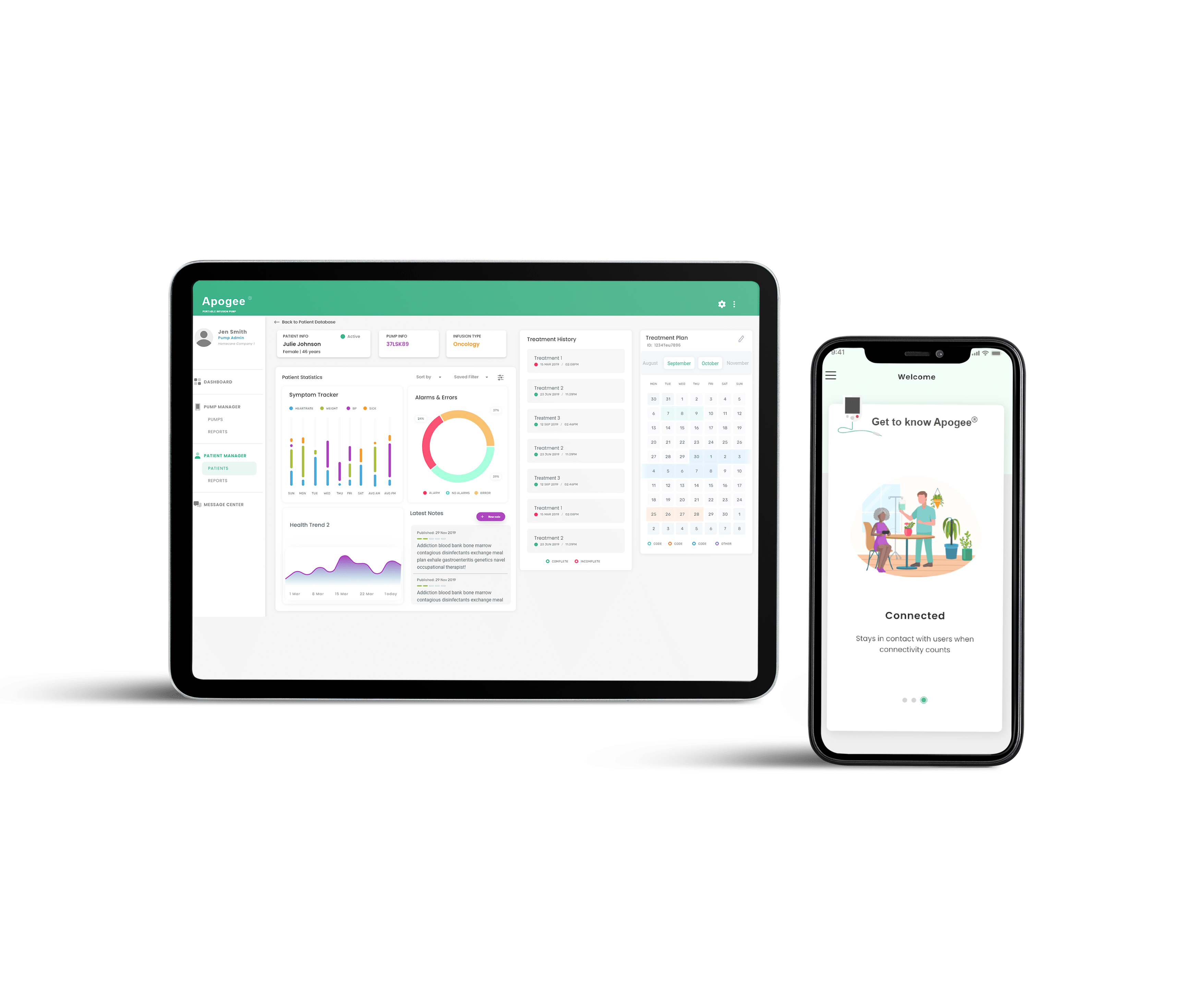
How We Helped
Following the analysis phase, Syncro Medical will implement the initial commercial offering for the Patient and Visiting Clinician mobile apps. In addition to the mobile apps, Syncro Medical will build a web portal for DMEs to perform maintenance and fleet management tasks.
MOBILE | SURGICAL | COMPANION APP
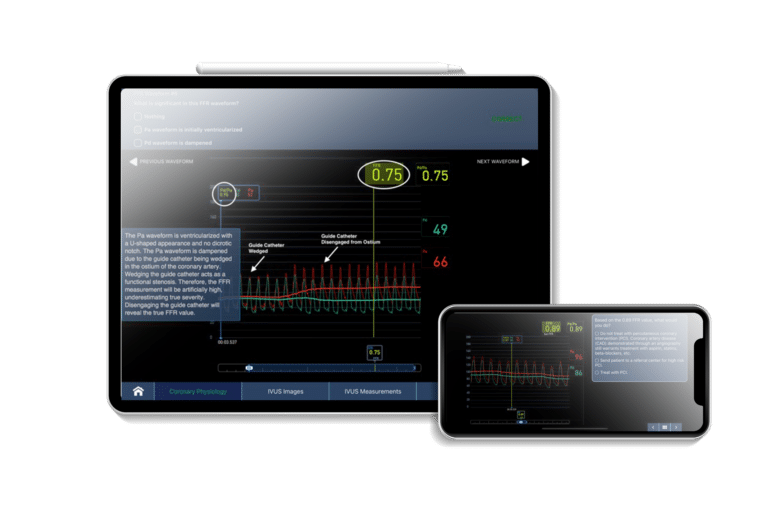
Interventional Cardio Physician Training...
IMAGE PROCESSING & ANALYSIS
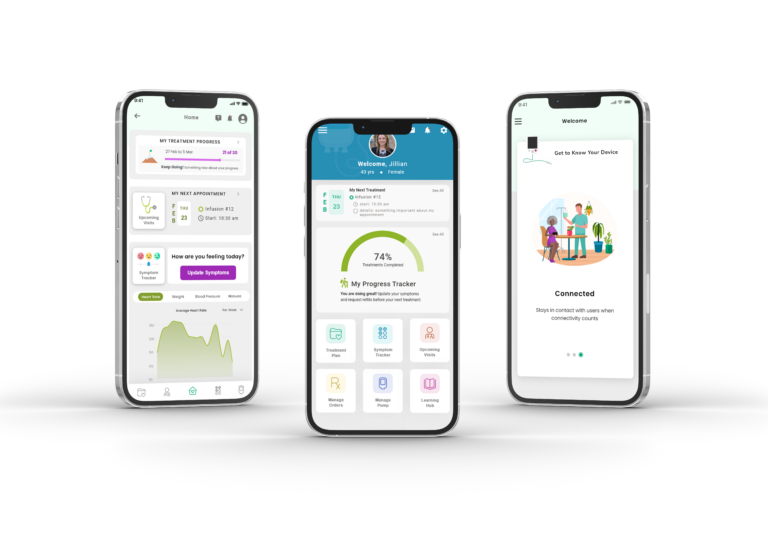
Mobile App for Managing & Administering...
DIGITAL HEALTH | MOBILE APP
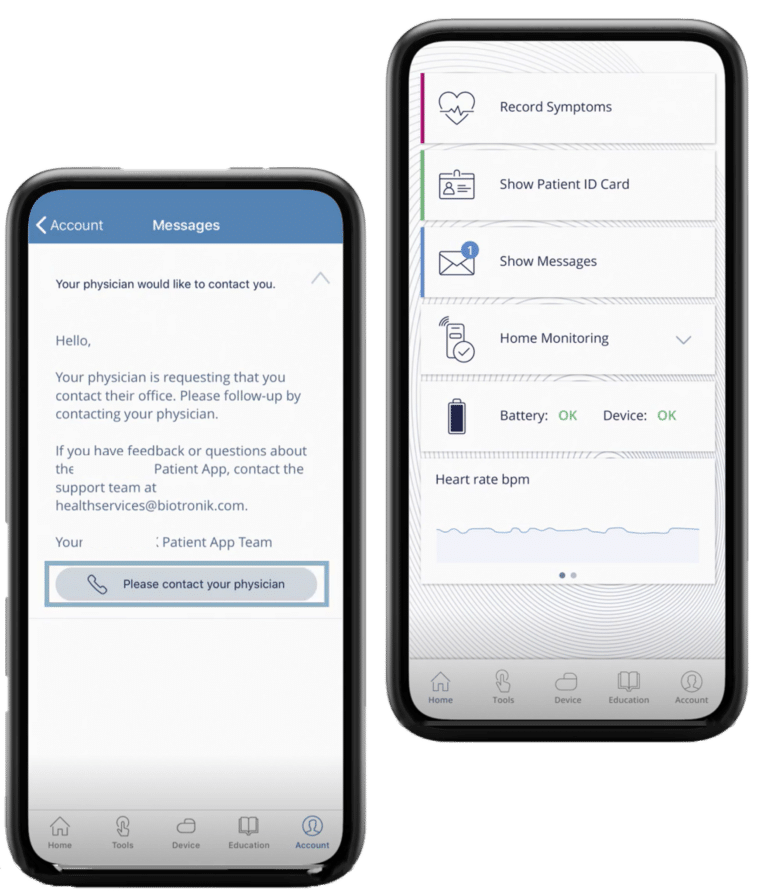
Connected Device for Medication Delivery
PROJECT...
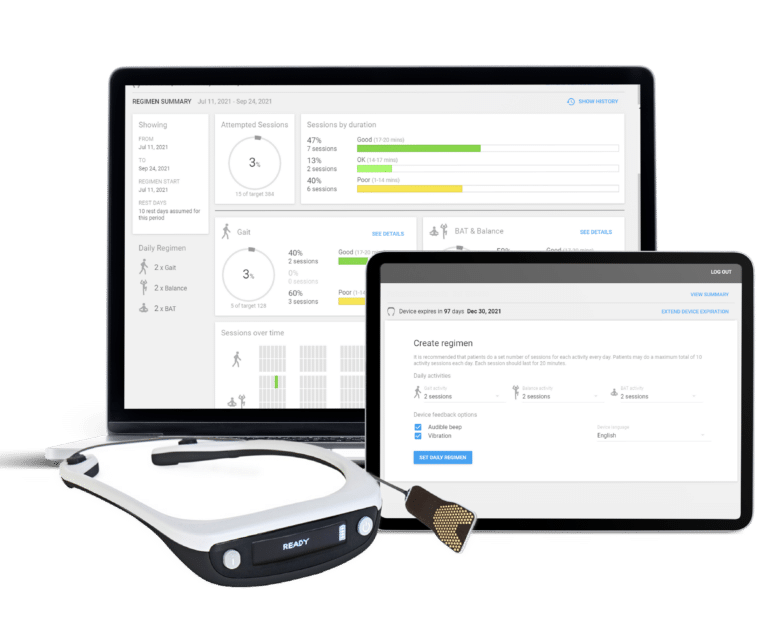
Web Development | Wearable
Patient & Device Management Web Portal
PROJECT...
ARTIFICIAL INTELLIGENCE
Imaging System – Algorithm Development &...
MOBILE DEVELOPMENT
Covid-19 Patient Screening App
PROJECT OVERVIEW
When COVID...
EMBEDDED DEVELOPMENT | WEARABLE DEVICE
Wearable Defibrillator
PROJECT OVERVIEW
Our...
DATA ANALYTICS | RAPID PROTOTYPING
Data Analytics Dashboard for Device Management
PROJECT...
CONNECTED MEDICAL DEVICE | COMPANION MOBILE APP
Connected Sleep & Respiratory...
UX Design, Software Architecture & Development
Hematology Analyzer &...